Share this
The Importance of Oxygen Safety & Eliminating Potential Ignition Sources
by Tristian McCallion on Wed, Dec 21, 2016 @ 13:12 PM
It's important to understand Oxygen enriched atmospheres to ensure safety.
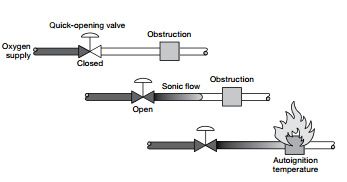
Above is an example of compression heating in an oxygen system that occurs when a valve is quickly opened and the gas stream compresses the oxygen downstream against an obstruction. For more information on oxygen system safety, download the catalogue.
There are three elements that are required to create a fire: fuel, ignition energy, and an oxidizer. When the oxygen is increased beyond the normal 21% level in the atmosphere, the risk of fire is greatly increased. It’s important to note that many materials that may not be combustible in a normal atmosphere will burn in an oxygen-enriched atmosphere. Combustible materials are easier to ignite and burn faster and hotter. Ignition sources that have no effect in air can be of critical importance in oxygen enriched systems.
Ignition Sources
Obviously, with all these issues, we must be extremely careful when dealing with oxygen enriched systems. A ‘Kindling Chain’ occurs when a small amount of energy ignites a material which creates a small fire. Once that fire is ignited, a chain reaction occurs to larger materials that generate more heat until the fire becomes self-sustaining. Because of this, every effort must be made to eliminate any potential cause of ignition. Some of the main ignition sources can include:
- Mechanical impact: When one object strikes another, heat is produced at the point of impact. The heat produced by a mechanical impact can act as an ignition source. For example, in an oxygen system, a mechanical component may break off and strike a pressurized container, producing heat upon impact. If the surface of the container is contaminated with oil, it can ignite and initiate the kindling chain sequence.
- Particle impact: Small particles can be carried along with a flowing oxygen stream, often at high velocity. When the particles strike a surface in the system, the impact energy is released as heat, and because of their small mass, the particles become hot enough to ignite larger materials.
- Friction: When two solid materials rub together, they generate heat which can ignite other materials
- Compression heating: When a gas flows through an orifice from high to low pressure, it expands and its velocity can reach the speed of sound. If the gas flow is blocked, it recompresses to its original pressure and becomes hot. The greater the pressure difference, the higher the gas temperature. This effect can be seen when pumping up a bike tire. As the pressure rises in the tire, the pump gets hot. In an oxygen system the oxygen temperature can be high enough to initiate the kindling chain. For this reason, fast opening valves should not be used in oxygen systems. Ball valves, for example, can give 80% flow when only 20% open. Slow opening valves should be used instead.
Risk management in oxygen systems
ASTM G128 discusses the hazards of oxygen service in much more depth and also gives design considerations and ignition sources in greater detail while G88 and Manual MNL36 provide specific design guidance. ASTM G4 Standards Technology Training course Controlling Fire Hazards in Oxygen Handling Systems provides detailed instruction in hazard analysis and risk management in oxygen systems.
In summary, the first and foremost rule for the safe use of oxygen is to consult an expert. Although oxygen systems present serious and unusual hazards, they are used safely throughout the industry because the risk of injury and economic loss can be managed and controlled.
More information on oxygen safety can be found in Swagelok’s Technical Bulletin Oxygen System Safety (MS-06-13)
Additional resources
- Are Your Fluid Systems Skating on Thin Ice?
- Seek Suppliers That Help You Prepare for Recovery
- Who Wouldn't Want to Find Something Shiny & Swagelok under the Tree?
In a hurry or have a question? Please click here to get in touch - we respond fast!
Or call 780-437-0640
Share this
- Local Services (103)
- Field Advisors (101)
- Training & Events (86)
- Fittings (81)
- Valves (66)
- Resources (62)
- Tubing (62)
- Sampling Systems (60)
- Design & Assembly (57)
- Resources - Downloads (40)
- Hose & Flexible Tubing (39)
- Frequently Asked Questions (37)
- Regulators (34)
- Cost Savings (33)
- Oil & Gas (33)
- Videos (33)
- Steam Systems (29)
- Mechanical Seal Support (17)
- Measurement Devices (15)
- Gas Distribution Systems (9)
- Rentals (6)
- winterization (6)
- Safety (5)
- Covid (3)
- Hydrogen & Clean Energy (3)
- About Us (1)
- April 2024 (3)
- March 2024 (2)
- January 2024 (3)
- December 2023 (2)
- November 2023 (3)
- October 2023 (2)
- September 2023 (3)
- August 2023 (3)
- July 2023 (3)
- June 2023 (2)
- May 2023 (4)
- April 2023 (2)
- March 2023 (2)
- February 2023 (3)
- January 2023 (2)
- December 2022 (1)
- November 2022 (1)
- October 2022 (2)
- September 2022 (5)
- August 2022 (3)
- July 2022 (6)
- June 2022 (4)
- May 2022 (3)
- April 2022 (1)
- March 2022 (2)
- February 2022 (3)
- January 2022 (4)
- December 2021 (4)
- November 2021 (6)
- October 2021 (3)
- September 2021 (5)
- August 2021 (9)
- July 2021 (5)
- June 2021 (7)
- May 2021 (7)
- April 2021 (4)
- March 2021 (3)
- February 2021 (3)
- January 2021 (2)
- December 2020 (3)
- November 2020 (3)
- October 2020 (2)
- September 2020 (3)
- August 2020 (3)
- July 2020 (3)
- June 2020 (3)
- May 2020 (3)
- April 2020 (2)
- March 2020 (3)
- February 2020 (3)
- January 2020 (3)
- December 2019 (2)
- November 2019 (3)
- October 2019 (3)
- September 2019 (2)
- August 2019 (3)
- July 2019 (2)
- June 2019 (2)
- May 2019 (3)
- April 2019 (3)
- March 2019 (3)
- February 2019 (2)
- January 2019 (3)
- December 2018 (2)
- November 2018 (2)
- October 2018 (4)
- September 2018 (2)
- August 2018 (3)
- July 2018 (3)
- June 2018 (2)
- May 2018 (4)
- April 2018 (3)
- March 2018 (3)
- February 2018 (3)
- January 2018 (4)
- December 2017 (1)
- November 2017 (4)
- October 2017 (4)
- September 2017 (4)
- August 2017 (5)
- July 2017 (3)
- June 2017 (4)
- May 2017 (4)
- April 2017 (3)
- March 2017 (5)
- February 2017 (4)
- January 2017 (4)
- December 2016 (3)
- November 2016 (3)
- October 2016 (4)
- September 2016 (3)
- August 2016 (4)
- July 2016 (2)
- June 2016 (2)
- May 2016 (2)
- April 2016 (4)
- March 2016 (2)
- February 2016 (3)
- January 2016 (4)
- December 2015 (4)
- November 2015 (4)
- October 2015 (5)
- September 2015 (2)
- August 2015 (4)
- July 2015 (5)
- June 2015 (2)
- May 2015 (3)
- April 2015 (5)
- March 2015 (3)
- February 2015 (4)
- January 2015 (3)
- December 2014 (5)
- November 2014 (4)
- October 2014 (4)
- September 2014 (4)
- August 2014 (4)
- July 2014 (5)
- June 2014 (4)
- May 2014 (4)
- April 2014 (5)
- March 2014 (4)
- February 2014 (4)
- January 2014 (4)
- December 2013 (3)
- November 2013 (4)
- October 2013 (5)
- September 2013 (4)
- August 2013 (5)
- July 2013 (4)
- June 2013 (3)
- May 2013 (4)
- April 2013 (5)
- March 2013 (2)
- February 2013 (3)
- January 2013 (5)
- December 2012 (3)
- November 2012 (3)
- October 2012 (5)
- September 2012 (3)
- August 2012 (4)
- July 2012 (4)
- June 2012 (1)



